ABOUT US
Discover Our Story Behind Advancing Pharma Innovation
Our Organization
Advancing Pharma Innovation with Science, Speed, and Integrity
At Santa Pharm, we are committed to advancing pharmaceutical innovation through high-quality, flexible, and science-led CDMO solutions. As a European CDMO with a global focus, we support the pharmaceutical industry across the entire product lifecycle — from early development to commercial manufacturing and distribution.
With decades of combined expertise in solid dosage forms and non-sterile liquids, we deliver customized support for formulation, analytical testing, regulatory compliance, and batch release. Our agile operations and client-first mindset ensure that every project is managed with transparency, technical integrity, and a shared mission: to bring better, safer medicines to patients — faster and more reliably.
Whether you're a start-up, biotech company, or established pharmaceutical manufacturer, Santa Pharm is your trusted partner from development to market.
Our Mission
Accelerating Success with Science-Driven Solutions
To deliver high-quality, science-driven pharmaceutical development and manufacturing solutions that accelerate our partners’ success and improve patient outcomes. We aim to serve as a seamless extension of our clients' teams — bringing deep technical expertise, regulatory insight, and a relentless commitment to quality at every stage.
Our Core Values
What Drives Us — Quality, Integrity, and Innovation
Quality First
We are uncompromising in our commitment to quality and compliance, aligned with international standards
Partnership
Every client is a long-term partner. We work collaboratively, with clear communication and mutual trust.
Scientific Integrity
We apply rigorous scientific methods and technical excellence from formulation to distribution.
Accountability
Our team takes full ownership of responsibilities, empowered to deliver results that meet our promises
Innovation
We invest in modern technologies and processes to meet today’s pharmaceutical challenges.
Respect & Responsibility
We act with ethics and respect — for our clients, teams, communities, and the patients who rely on us.
Our Vision
Empowering Global Access to Safe and Effective Medicines
At Santa Pharm, our vision is to be a trusted European leader in pharmaceutical development and manufacturing — recognized globally for our innovation, scientific excellence, and unwavering commitment to quality.
We envision a future where every patient, no matter where they live, has timely access to safe, effective, and affordable medicines. To achieve this, we aim to empower our partners — from early-stage biotech to global pharmaceutical companies — with the tools, technologies, and expertise needed to navigate complex challenges and bring breakthrough therapies to market.
Through continued investment in people, processes, and digital innovation, we strive to set new standards for reliability, transparency, and performance in the CDMO industry — creating long-term value for our clients and better outcomes for patients worldwide.
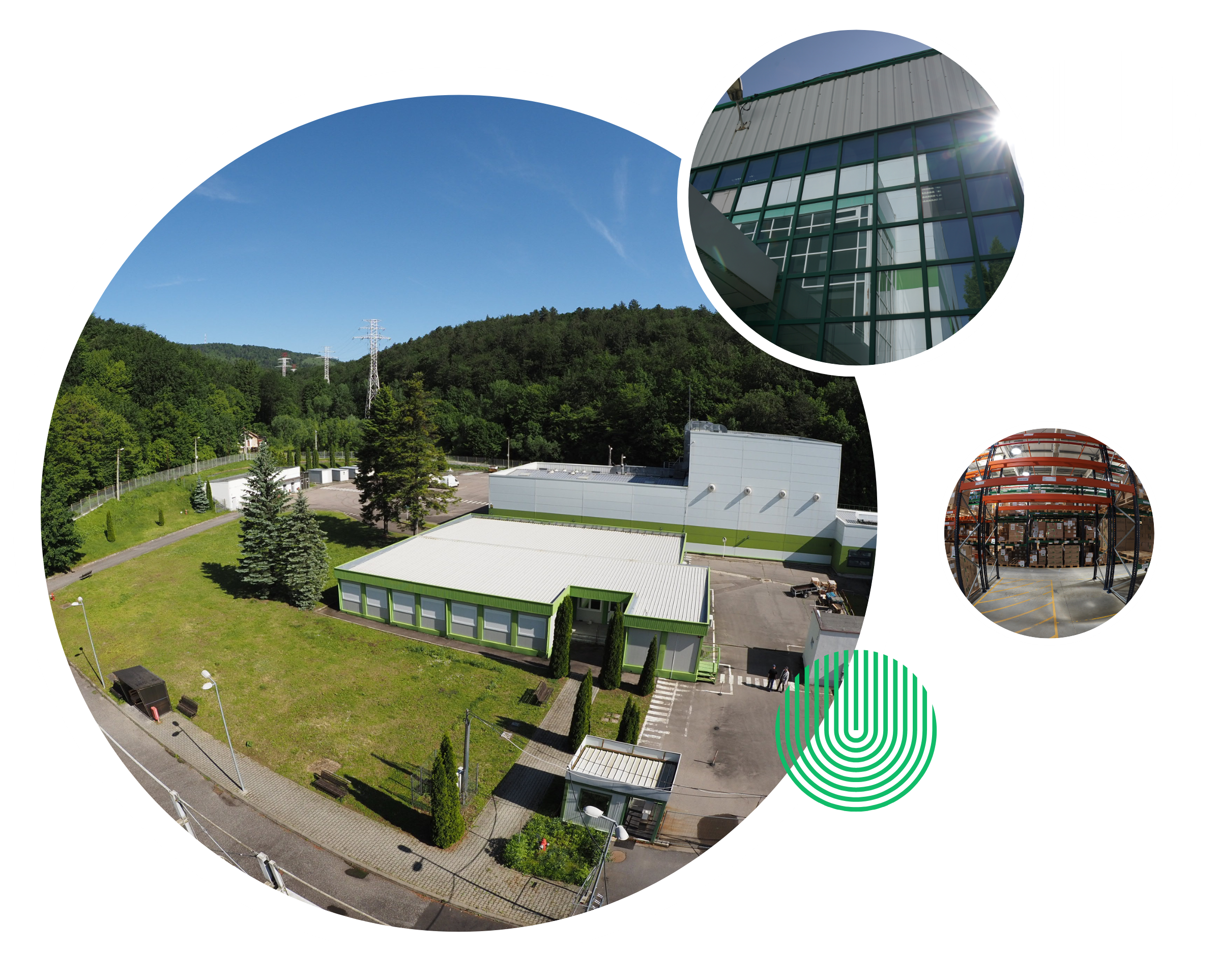
Certifications & Compliance
Globally Certified. Fully Compliant. Always Audit-Ready
At Santa Pharm, compliance isn’t just a requirement — it’s a cornerstone of our operation. We are fully aligned with EU-GMP standards and are regularly audited by European regulatory authorities. Our systems are built to ensure traceability, safety, and ongoing regulatory readiness
Regulatory Readiness
Prepared for Inspections. Focused on Excellence.
Santa Pharm operates under a robust Quality Management System (QMS) that ensures continuous regulatory compliance and audit readiness. Our regulatory and quality teams proactively manage certifications, support client audits, and respond to changing industry requirements — providing confidence, clarity, and peace of mind at every stage of your product’s journey.
